At present there is no agreement in the scientific literature on the weight of somatic, cognitive and emotional clusters in a mood disorder diagnosis. In particular, according to Clark and colleagues, the traditional emphasis on somatic and behavioural symptoms is insufficiently supported by empirical evidence (Clark, Beck, & Alford, 2001). Indeed, many studies have demonstrated that, in order to formulate a major depression diagnosis, subjective symptoms like depressed mood, desperation and self-devaluation are just as important, if not more so, as biological symptoms like weight loss or insomnia (Coulehan, Schulberg, Block, & Zettler-Segal, 1988; Lovibond & Lovibond, 1995). Anedonia is also considered a typical mood depression symptom (Clark & Watson, 1991).
A research on day-hospital psychiatric patients (52% with clinically relevant mood depression) and healthy subjects demonstrated that items of the Beck Depression Inventory (BDI) relating to cognitive and subjective symptoms saturate the depression factor in factorial analysis, whereas biological symptoms like the loss of weight, appetite or interest in sex do not show factorial significant weight (Beck, 1967; Clark, Beck, & Beck, 1994; Steer, Clark, Beck, & Ranieri, 1995).
Investigating the frequency of these symptoms in day-hospital patients with major depressive episodes, Buchwald and Rudick-Davis demonstrated that sleep disturbances, as well as loss of energy and appetite, are detected in over 80% of cases, whereas psychomotor alterations and self-devaluation are relevant in less than 70% of depressed patients (Buchwald & Rudick-Davis, 1993).
Overall, similar data show a general disagreement in the scientific literature over the importance of biological symptoms for the definition of a mood disorder diagnosis in the psychiatric population. In particular, there are two main opinions on the weight of somatic symptoms: that they should be considered an expression of the mood disorder (Buchwald & Rudick-Davis, 1993) or that they are just a secondary expression of the disturbance itself (Clark et al., 1994; Steer et al., 1995).
The question of somatic symptoms becomes very important for the comorbidity between mood depression and somatic diseases, when physical symptoms demonstrate both somatic and emotional pathogenesis, as is the case with pain and fatigue in oncological patients (Duddu, Isaac, & Chaturvedi, 2003).
In other words, there is at present an absence of agreement over the effective importance of the somatic cluster symptoms in the definition of a mood disorder diagnosis in oncological patients or in comorbidity with another severe organic pathology. In fact, although the traditional distinction of depressive clusters as affective, cognitive and somatic is more or less widely accepted by the scientific community for psychiatric patients, it is less well-defined as far as patients in comorbidity with other organic pathologies are concerned (Akechi et al., 2003; Reuter, Raugust, Bengel, & Harter, 2004).
Indeed, in such patients the role of somatic symptoms (loss of appetite, weight or energy, fatigue, sleep disturbances) is much less clear, since it is possibly secondary to the pathology itself or its medical treatment (radio and/or chemotherapy) rather than due to a mood disorder.
However, this aspect is extremely important when psychiatrically diagnosing or psychologically supporting oncological patients: by considering somatic symptoms from the perspective of a mood disorder diagnosis the risk is that of obtaining many false positive diagnoses, whilst by taking the opposite perspective, the risk is that of obtaining many false negative cases, with evident side effects on clinical practice.
The role of physical symptoms in mood depression in comorbidity with organic pathologies has not yet been defined: some authors (Cohen-Cole, Brown, & McDaniel, 1993) hold that no somatic symptom should be used as a diagnostic criterion (exclusive approach); others (Creed, 1997) consider that only those symptoms which cannot be ascribed to the pathology or its treatment (aetiological approach) should be considered. Research, however, has clearly shown that these approaches produce a high number of false negative diagnoses advising against their clinical use.
Another approach has been proposed: the so-called “substituting approach”, which suggests the substitution of somatic symptoms by affective and cognitive ones (Endicott, 1984). This approach guarantees better results, although the criterion of substitution on which the whole procedure is based has not yet been validated.
Moreover, even the most recent clinical studies do not agree on the usefulness of physical symptoms to express a mood disorder diagnosis in psychoncology (Akechi et al., 2003; Reuter et al., 2004). Based on these considerations, the principal aim of this study is to investigate the weight of somatic, affective and cognitive symptoms in a mood disorder diagnosis in oncological patients.
Methods
Patients
The sample consisted of 151 in-patients of the Surgical Oncology Unit of S. Giovanni Battista Hospital in Turin, Italy. The patients enrolled in the study were evaluated in the period September 2010 - September 2011 by an experienced psychoncologist, after their first medical examination at the day hospital of the same department. Before the psychological assessment, demographic data were collected through a semi-structured interview at the clinic, conducted by the same experienced psychoncologist. All the questionnaires were completed at the clinic.
The sample consisted of 84 males and 67 females with a Mean (SD) age of 49.3 (SD = 23.5) years. The patients were on the waiting list for surgical intervention and affected, respectively, by colon-rectal (32%), breast (26%), gastric (21%), ovarian (11%), or lung (10%) cancer (Table 1).
Table 1
Demographic and Clinical Characteristics of the Patients
| Sex | ||
|---|---|---|
| Male | 84 | 55,6% |
| Female | 67 | 44,4% |
| Age | ||
| Mean | 49,3 | |
| Range | 30-84 | |
| SD | 23,5 | |
| Marital status | ||
| Married | 118 | 78,1% |
| Widowed | 14 | 9,4% |
| Single | 13 | 8,7% |
| Divorced | 6 | 3,8% |
| Disease site | ||
| Colorectal | 48 | 32,0% |
| Breast | 39 | 26,0% |
| Gastric | 32 | 21,0% |
| Ovarian | 17 | 11,0% |
| Lung | 15 | 10,0% |
| Extent of disease | ||
| Local | 89 | 58,8% |
| Loco-regional | 54 | 35,8% |
| Methastatic | 8 | 5,4% |
Materials and Procedure
The patients were evaluated with the following rating scales:
HADS (Hospital Anxiety and Depression Scale) (Zigmond & Snaith, 1983), a self-evaluating scale consisting of two seven-item sub-scales, assessing anxiety and depression. It is specific for patients with organic pathologies, since it does not consider somatic symptoms (cephalea, weight loss, insomnia, etc.) which could be confused with symptoms due to the disease or the treatment itself. This scale has been validated for a hospital environment and is widely used with oncological patients. Total scores vary from 0 (no distress) to 42 (maximum distress). In the present study, a cut-off of ≥ 8 for both sub-scales was adopted, as in the case in most researches on depression in organic comorbidity, in order to detect also subsindromic depressive disturbances (Castelli, Binaschi, Caldera, & Torta, 2009).
MADRS (Montgomery Asberg Depression Rating Scale) (Montgomery & Asberg, 1979), a hetero-evaluating scale for the assessment of depression, comprising 10 items with a score ranging from 0 to 6; it investigates symptoms such as depressed mood, inner tension, sleep disturbances, loss of appetite, difficulty in concentration, asthenia, anhedonia, pessimistic and suicidal thoughts. The sum of the scores is an expression of general pathology severity. Several studies have focused on the issue of establishing the most appropriate cut-off rate for the presence/absence of depression in comorbidity: most agree that a cut-off of ≥ 10 can be considered appropriate (Zimmerman, Chelminski, & Posternak, 2004). Similarly, a cut-off of ≥ 35 is widely accepted for the detection of severe depressive syndrome, as suggested by the authors and confirmed by subsequent studies (Müller, Szegedi, Wetzel, & Benkert, 2000). According to the literature, three different sub-groups can therefore be defined: absence of depression (0-9), mild-moderate depression (10-34), severe depression (35-60). This scale suited our research particularly well since the ten items can be grouped following the somatic, affective and cognitive clusters, allowing separate statistical analyses.
As already found in literature (Reuter et al., 2004), the subdivision is made according to three clusters of items:
-
behavioural, referring to “apparent sadness”, “reported sadness”, “inner tension”, “inability to feel” (anhedonia)
-
somatic, including “reduced sleep” “reduced appetite” and “lassitude” (fatigue)
-
cognitive, including “difficulty in concentration”, “pessimistic thoughts”, “suicidal thoughts”
The MADRS, filled out by an expert psychoncologist was used together with the self-evaluative HADS.
Statistical Analysis
Means and standard deviations were calculated for the abovementioned rating scales. Mean values were compared with the independent t test procedure (two-tailed), using the statistical package for the social sciences [SPSS 15.0]. p values < 0.05 were considered statistically significant.
Results
HADS
57,4% of group scored above the cut-off (≥ 8) for depression and 42,6% below. 47,3% scored above the cut-off (≥ 8) for anxiety, while 52,7% showed no specific anxiety symptom.
MADRS
At MADRS global scores, 44% of patients scored between 0-9, indicating absence of depression; 43.7% scored between 10-34, indicating mild/moderate depressive symptoms; 12.3% were in the range 35-60, indicating severe mood depression. These data are shown in Figure 1.
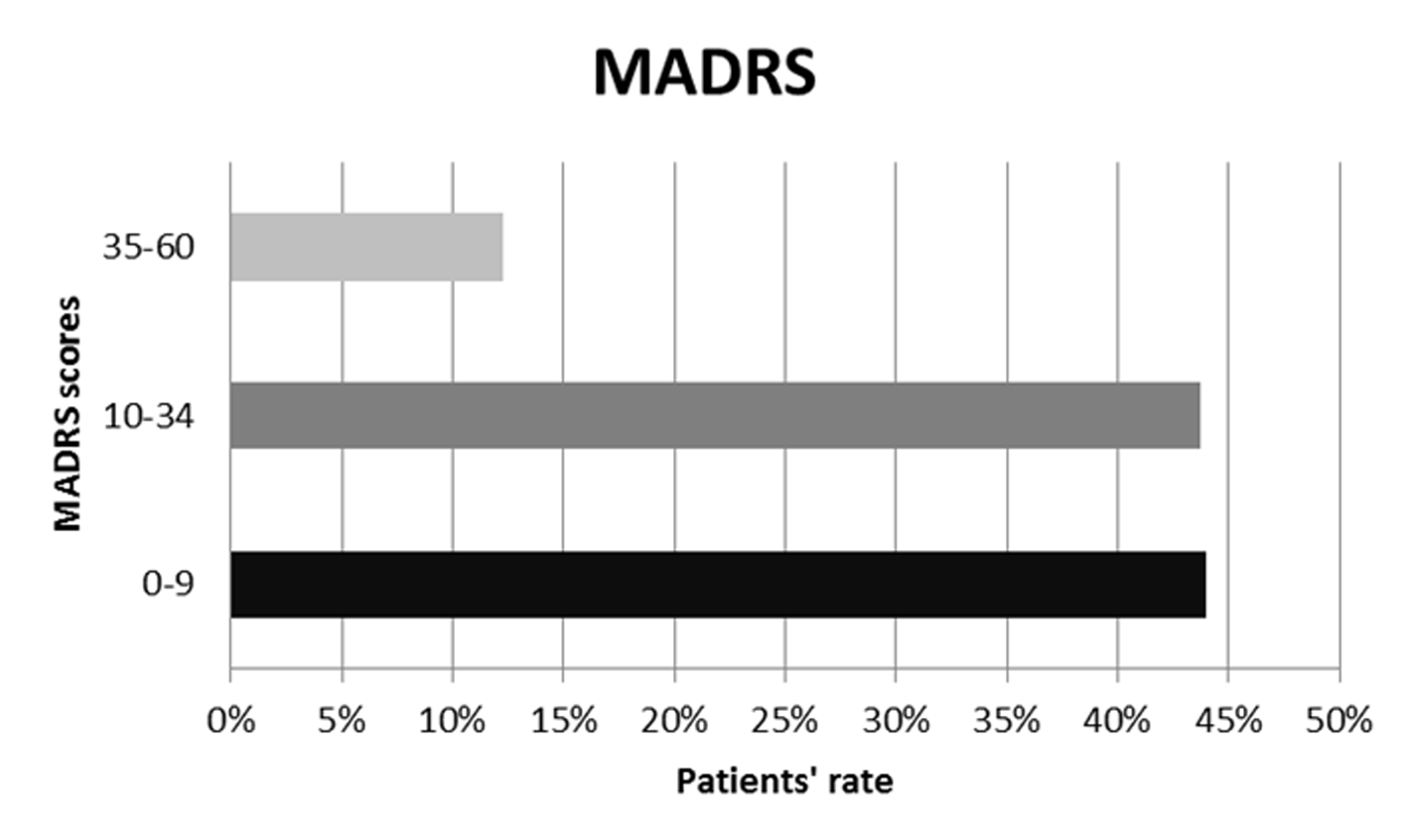
Figure 1
MADRS global scores.
To evaluate the diagnostic relevance of the affective, somatic and cognitive clusters, the global MADRS score for each single cluster (obtained by adding all the cluster’s item scores) was considered separately for the group of patients above the cut-off at the HADS-D and for the group below, because HADS does not consider the somatic symptoms of depression (see Figure 2).
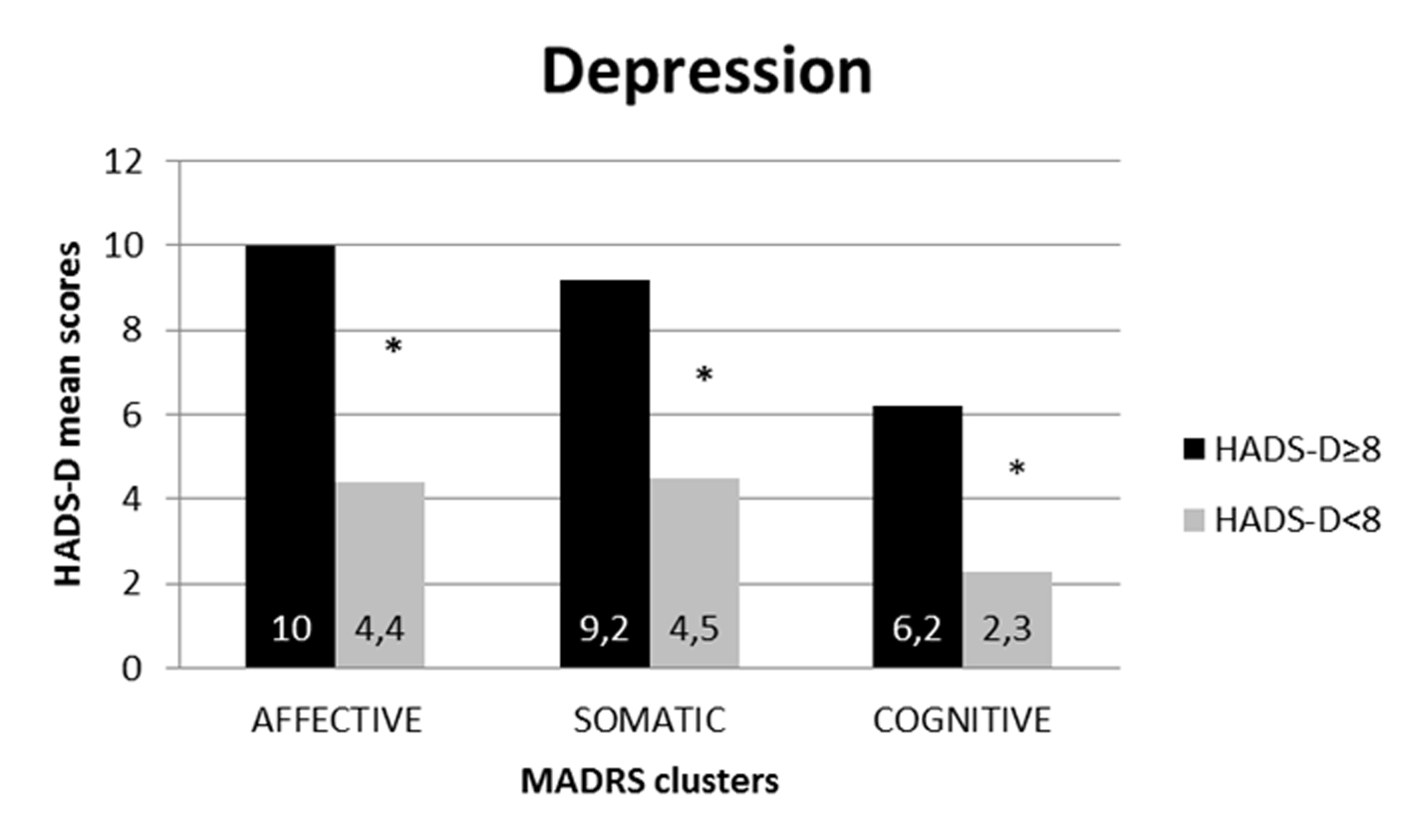
Figure 2
MADRS's clusters distribution according to HADS-depression cut-off.
*p < .001.
The population with HADS-D above cutoff (8) shows higher mean values in all symptoms clusters of MADRS than the population below cut-off (p < .001): affective (mean values 10 ± 2,98 vs. 4,4 ± 2,34), somatic (mean values 9,2 ± 2,12 vs. 4,5 ± 2,15), and cognitive (mean values 6,2 ± 1,85 vs. 2,3 ± 1,2).
Following this, the score difference between the two groups was statistically analyzed. This analysis showed how scores at all three clusters on the MADRS are significantly higher (p < 0.01) in the group of patients above the cut-off (≥ 8) on the HADS-D subscale.
The same procedure was used to evaluate the trend of the three clusters on the HADS-A (anxiety) subscale (see Figure 3).
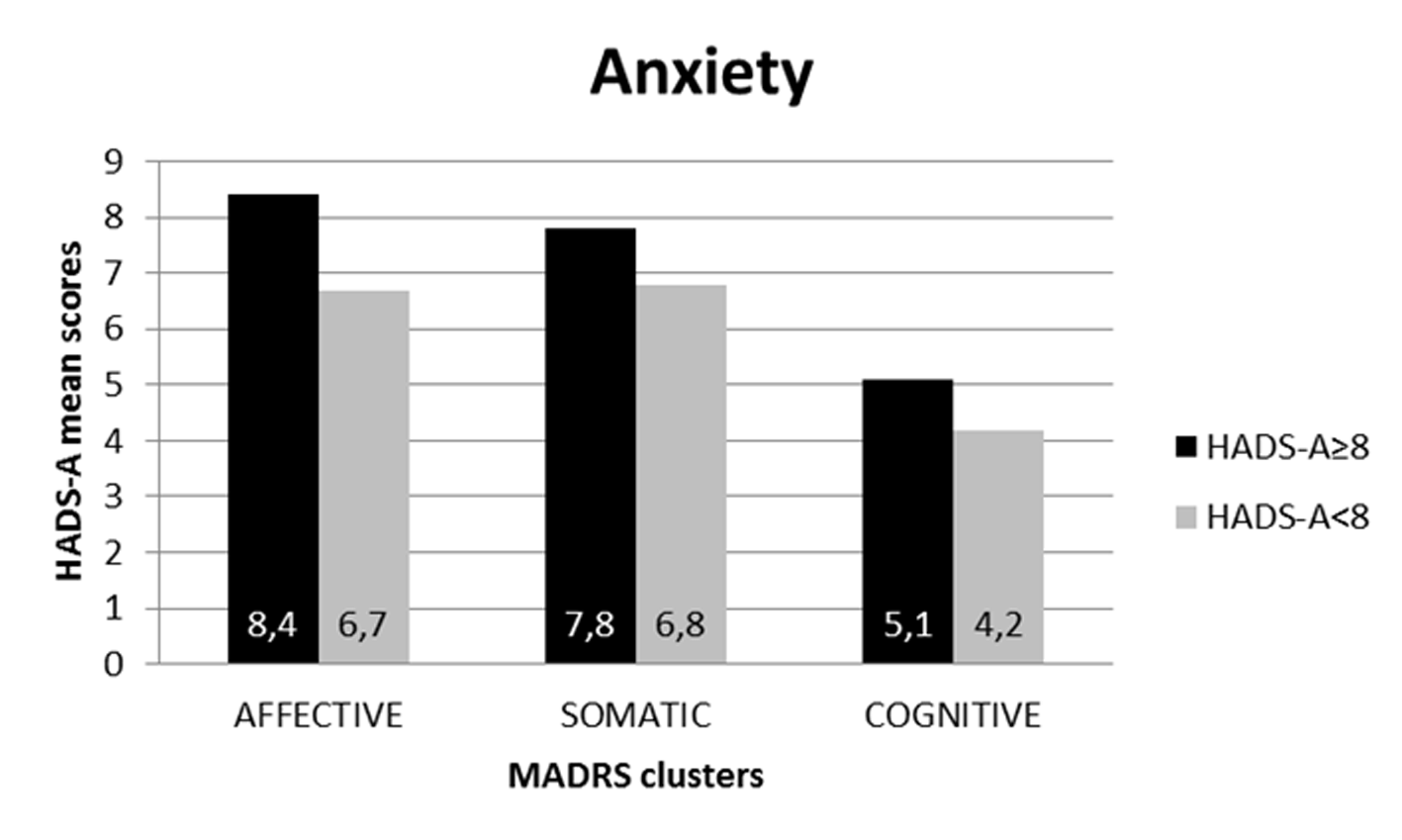
Figure 3
MADRS's clusters distribution according to HADS-anxiety cut-off.
The population with HADS-A above cutoff (8) shows higher mean values in all symptoms clusters of MADRS than the population below cut-off: affective (mean values 8,4 ± 3,58 vs. 6,7 ± 4,20), somatic (mean values 7,8 ± 2,19 vs. 6,8 ± 3,07), and cognitive (mean values 5,1 ± 1,91 vs. 4,2 ± 1,97). In this case, however, the scores at each MADRS cluster did not show statistically significant differences between the two HADS-A groups.
The data also show how the trend of clusters on MADRS scores is not homogenous. Comparing the MADRS scores between the two groups (above and below the cut-off point of HADS-D), more relevant differences were observed in the affective and somatic clusters than in the cognitive cluster (see Figure 2).
Discussion
The aim of this study was to evaluate the relevance of somatic, affective and cognitive clusters in mood disorders in oncological comorbidity. Results suggest that somatic symptoms can actually be read as signals of mood disturbances also in patients with comorbidity.
An analysis of MADRS scores showed that: 44% of patients scored 0-9, thus showing no depressive symptom; 43.7% scored 10-34, thus satisfying the criteria for mild/moderate depression; and 12.3% scored ≥ 35, with severe depressive symptoms. Overall, 56% of patients exceeded the MADRS cut-off and 57.4 of patients exceeded the cut-off on the HADS-D (depression) subscale, confirming our previous data on the reliability of HADS as an effective screening tool (Castelli et al., 2009). A HADS cut-off score of 8, which is lower than the value used in psychiatric population, was chosen in order to identify sub-syndromic mood disturbances that represent a high risk of development of clinical depression.
As already mentioned, the evaluation of the relevance of somatic, affective and cognitive clusters in mood disorders in oncological comorbidity led to some interesting considerations. First of all, the three clusters showed significantly higher scores (p < 0.01) in patients with HADS scores above the cut-off for depression, compared to patients below. This suggests that for oncological patients, too, all clusters are useful in the process of mood disorder diagnosis.
Furthermore, the relevance of each cluster is different. Analyzing the single clusters, it could be noticed how the affective cluster showed the highest mean score among the group of patients with HADS-D scores above the cut-off rate according to the literature. The somatic cluster is next in terms of relevance, demonstrating very high scores in patients with depressive symptoms and significantly lower scores in the “not depressed” group.
Whilst the cognitive cluster has lower scores than the first two, it shows a significant difference between the sub-groups above and below the cut-off.
However, the data nevertheless confirmed that both affective and somatic symptoms are relevant for mood disorder in oncological patients.
Indeed, if somatic symptoms were to be considered exclusively due to the cancer or its treatment, and were not linked to mood aspects, then the cluster scores should be similar in the two groups (scores above and below the cut-off rates). The data thus suggest that in this population physical symptoms are also linked to mood disturbances.
Physical symptoms should thus be considered the result of both organic and emotional components in oncological patients. In other words, appetite, pain, fatigue, sleep and sexuality obviously demonstrate a co-occurrence of emotional and somatic aspects that cannot be evaluated from a dichotomic organic or emotional viewpoint.
This is clearly not to insinuate that, for example, reduced appetite in cancer patients is caused exclusively by mood disorder and is not a consequence of the disease or chemotherapy, but to affirm that such a disturbance is experienced more intensively by oncological patients who are depressed than by those who are not.
The relevance of somatic symptoms of mood depression is also related to the question of systemic disease (Torta, 2002): the pathogenesis of a mood disorder is due not only to neurotransmitter imbalance, but also to broader modifications in immune and hormonal systems (such as increased pro-inflammatory cytokines and cortisol). Somatic symptoms thus demonstrate a pathogenetic component induced by mood depression itself. Furthermore, patients with a chronic disease can find a somatic way to express their emotional disease instead of verbal or non-verbal communication (secondary alexithimia).
Mood disturbance seems to induce oncological patients to express somatic disturbances significantly more than “healthy” patients (secondary alexythymia); in practice, this means that somatic symptoms can be read as signals for mood disturbances in patients in comorbidity, too.
According to recent literature, it could be hypothesized that alexythymic subjects have difficulty identifying and expressing internal emotional states and distinguishing between feelings and somatic sensations of emotional arousal (Duddu et al., 2003).
This hypothesis is supported by the data on the trend of the same three clusters of the HAD – anxiety subscale. In fact, on the HAD-A subscale none of the three clusters shows significantly different scores in the groups of patients above and below the cut-off. Whilst these data are more comprehensible with regard to the cognitive and affective clusters (less correlated to anxiety syndromes), they appear very revealing as far as the somatic cluster is concerned.
If the expression of a somatic symptom in oncological patients were to refer exclusively to generic psychological distress and not directly to the depressive spectrum, we should be able to observe a similar trend in the anxiety subscale as well, that is, significantly higher scores in the group above the cut-off of the HADS-A (anxiety) subscale with respect to the group below.
The present results disagree with the exclusive approach suggested by other authors. Wedding and colleagues concluded that somatic symptoms in depressed cancer patients have to be excluded from diagnostic criteria for depressive disorders, since they seem related not to depression but to the severity of the underlying disease (Wedding et al., 2007). However, independently of our aforementioned considerations on the links between emotional and organic pathogenesis in somatic symptoms of depression, we partially disagree with the instrument used for the evaluation of somatic symptoms in the study performed by Wedding and colleagues. In that study, indeed, the somatic symptoms were investigated by BDI, which shows some limits in the oncological population. The somatic cluster, in fact, includes cognitive symptoms of depression, such as change of body image and hypochondria. Besides, BDI is mainly focused on the subjective perception of symptoms. Moreover, the study compares depressive symptoms experienced by the healthy general population and by the oncological population, without considering that some cancer patients, who have difficulty identifying and expressing internal emotional states (secondary alexithymia) could express their psychological distress by an increase in the number of complaints related to physical symptoms.
The present study is limited by the relatively small number of patients and the lack of a control group; further, wider and longitudinal studies including a larger number of diagnostic tools (DSM IV-TR citeria) are needed to confirm the reliability of our findings. Although not exhaustive, the results emerging from this study confirm the role of the soma in the perception and in the expression of psychological suffering in cancer patients.
 This is an open access article distributed under the terms of the Creative Commons
Attribution License (
This is an open access article distributed under the terms of the Creative Commons
Attribution License (